Principle:
Ultraviolet-visible spectroscopy (UV/VIS spectroscopy) defines the spectroscopy of photons in the UV-VIS region of electromagnetic spectra. Light energy excites electrons from ground states to excited states. A UV/VIS/NIR Spectrophotometer measures the amount of light transmitted through a sample by ratioing the intensity light (Io) to the intensity of the transmitted light (I) in the UV/VIS and near infrared regions. For quantification of unknown samples, absorbance is often measured using Beer Lambert’s Law. The law states that the amount of light absorbed by the sample is directly proportional to its concentration.
Current model:
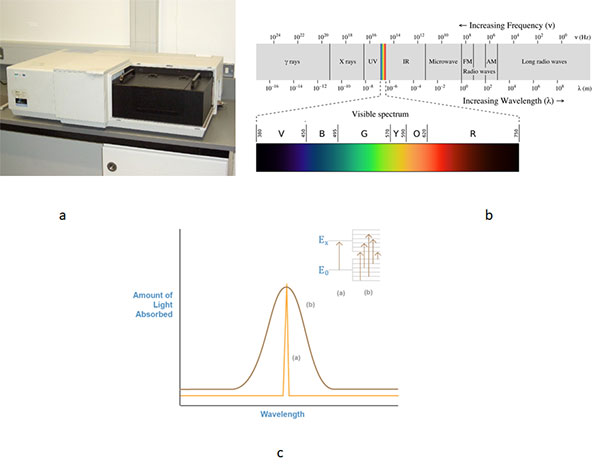
a (Perkin Elmer Lambda 900 UV/VIS/NIR Spectrometer), b (Electromagnetic radiation), and c (graph of light absorbed of sample vs. Wavelength in nm where Ex and Eo represent the electronic energy levels)
See video for further information
Spectrometer
The Perkin Elmer Lambda 900 UV/VIS/NIR spectrometer is a double-beam, double monochromator ratio recording system with pre-aligned tungsten-halogen and deuterium lamps as sources. The wavelength is from 175 to 3300nm with an accuracy of 0.08 nm in the UV-VIS region and 0.3nm in the NIR region guaranteed. It has a photometric range of +/- 6 in absorbance mode. The available accessories include a 60mm Spectralon coated integrating sphere with a range from 200 to 2500 nm for measurement of samples of high optical density. A common-beam depolariser for measurements with depolarised light in the 190 to 2600nm range is also available.
Technical Specifications:
Wavelength Range: 190 - 3300 nm
Accuracy: +/- 0.08 nm UV/VIS
+/- 0.30 nm NIR
Photometric Range: +/- 6 A
Photometric Accuracy: +/- 0.003 A at 1 A
Integrated sphere Option
Range: 200 - 2500 nm
Common Beam Depolarizer
Range: 190 - 2600 nm
Double Beam Polarizer (Depolarizer)
Range: 300 - 2600 nm (190 - 2600 nm
Typical samples:
Liquids and solutions are used efficiently for UV/VIS spectrometer. The sample may scatter the light more than absorb it if the analyte is suspended with solids in a liquid.
